ALPHA3
NOW ENROLLING
The ALPHA3 trial aims to embed investigational allogeneic CAR T as part of the first‑line (1L) treatment regimen in LBCL
CEMACABTAGENE ANSEGEDLEUCEL (CEMA-CEL) IS AN ALLOGENEIC CAR T PRODUCT BEING STUDIED AS PART OF 1L TREATMENT FOR PATIENTS WITH LBCL WHO ARE LIKELY TO RELAPSE
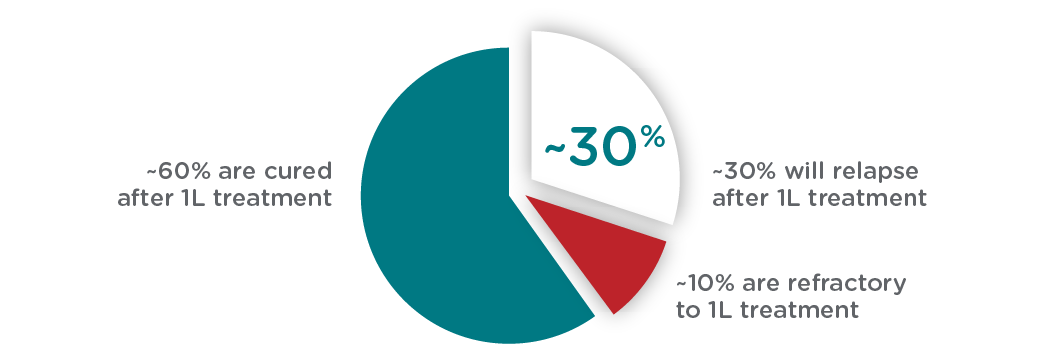
Over 60,000 patients are expected to be treated for LBCL annually in the US, the EU, and the UK. While first line (1L) chemoimmunotherapy (R-CHOP) is effective in most patients, ~30% will relapse and require subsequent treatment. Currently, no diagnostic tests exist to identify those patients whose disease will relapse.
AMONG PATIENTS TREATED WITH R-CHOP
ALPHA3 STUDY DESIGN OFFERS UNIQUE FEATURES

FIRST PROSPECTIVE TRIAL TO INCORPORATE INVESTIGATIONAL FORESIGHT CLARITYTM MRD TEST
The presence of minimally residual disease (MRD) remaining after therapy is predictive of relapse, yet currently there are no tests available that have the accuracy/performance to reliably detect residual disease at the end of 1L treatment in LBCL. The ALPHA3 trial will screen patients who are likely to relapse after 1L treatment for enrollment in the trial with the Foresight CLARITYTM Investigational Use Only (IUO) MRD test, powered by PhasED-SeqTM, which recently received Investigational Device Exemption (IDE) from the U.S. Food and Drug Administration (FDA).
Leveraging CLARITY’s ultra-sensitive MRD technology, cema-cel will be administered as a one-time infusion immediately upon detection of MRD at the completion of six cycles of R-CHOP or other standard 1L chemoimmunotherapy regimen.
STUDY DETAILS
The pivotal Phase 2 ALPHA3 trial (N=240) will leverage a state-of-the-art, ctDNA-based investigational minimal residual disease (MRD) assay to identify patients whose disease is likely to relapse and randomize against the current standard-of-care (“watch-and-wait”) v. a single infusion of cema-cel.
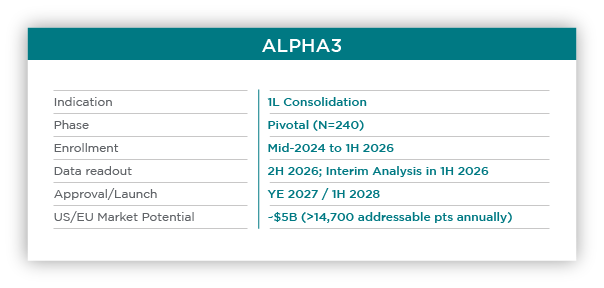
Data from the pivotal Phase 2 study is planned for YE 2026. The successful outcome of ALPHA3 is expected to be the basis for a 2027 BLA submission and 2028 US launch. Cema-cell in 1L consolidation LBCL can potentially change how patients with LBCL are managed and provide revenue potential of ~$5B in the US, EU and UK.
Allogene has oncology rights to cema-cel in the US, EU and UK with options in China and Japan.
DATABRIEF VIDEO FOR ALPHA3
R-CHOP, rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone.
