AlloCAR T™
ALLOGENEIC CAR T —
THE NEXT REVOLUTION IN CELL THERAPY
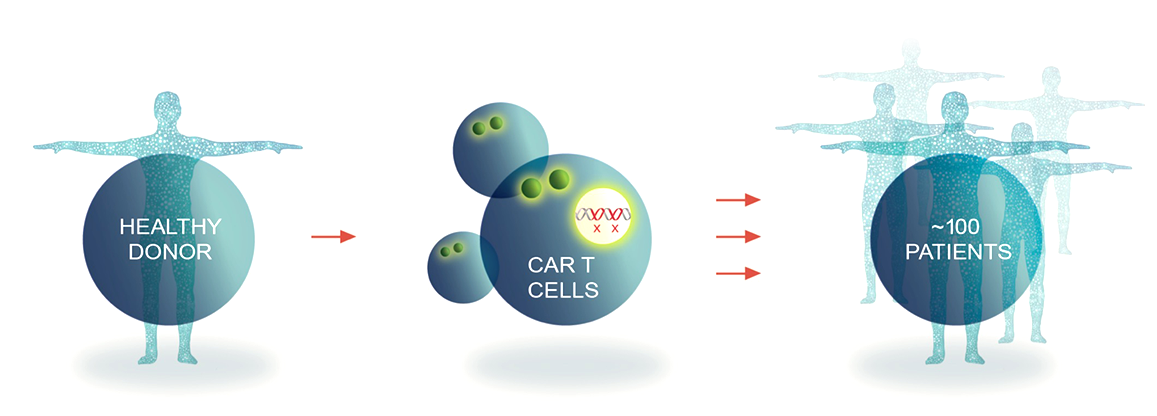
Allogene is working to overcome the limitations of autologous CAR T therapies by creating investigational allogeneic CAR T cell products, or AlloCAR T™ products. Unlike autologous cell therapy, AlloCAR T™ products use T cells from healthy donors.
These cells are isolated in a manufacturing facility, engineered to express CARs to recognize and destroy cancer cells, and modified via gene editing to limit autoimmune response when given to a patient. These products are then stored for off-the-shelf use on demand. We believe that, at scale, a single manufacturing run has the potential to yield treatment for 100+ patients.
WHY ALLOGENEIC CELL PRODUCTS
HAVE THE POTENTIAL TO LEAD THE REVOLUTION

ACCESS
- Potential to treat all eligible patients
- Repeat dosing, if needed
- No need for complex logistics

COST
- Scalable and efficient manufacturing
- Potential to treat 100+ patients from a single manufacturing run
- Lower ancillary costs

SPEED/RELIABILITY
- Off-the-shelf for on-demand treatment
- Less product variability, made from healthy T cells

INNOVATION
- Multiplex gene-engineering and gene-editing capabilities
- Opportunity for product optimization
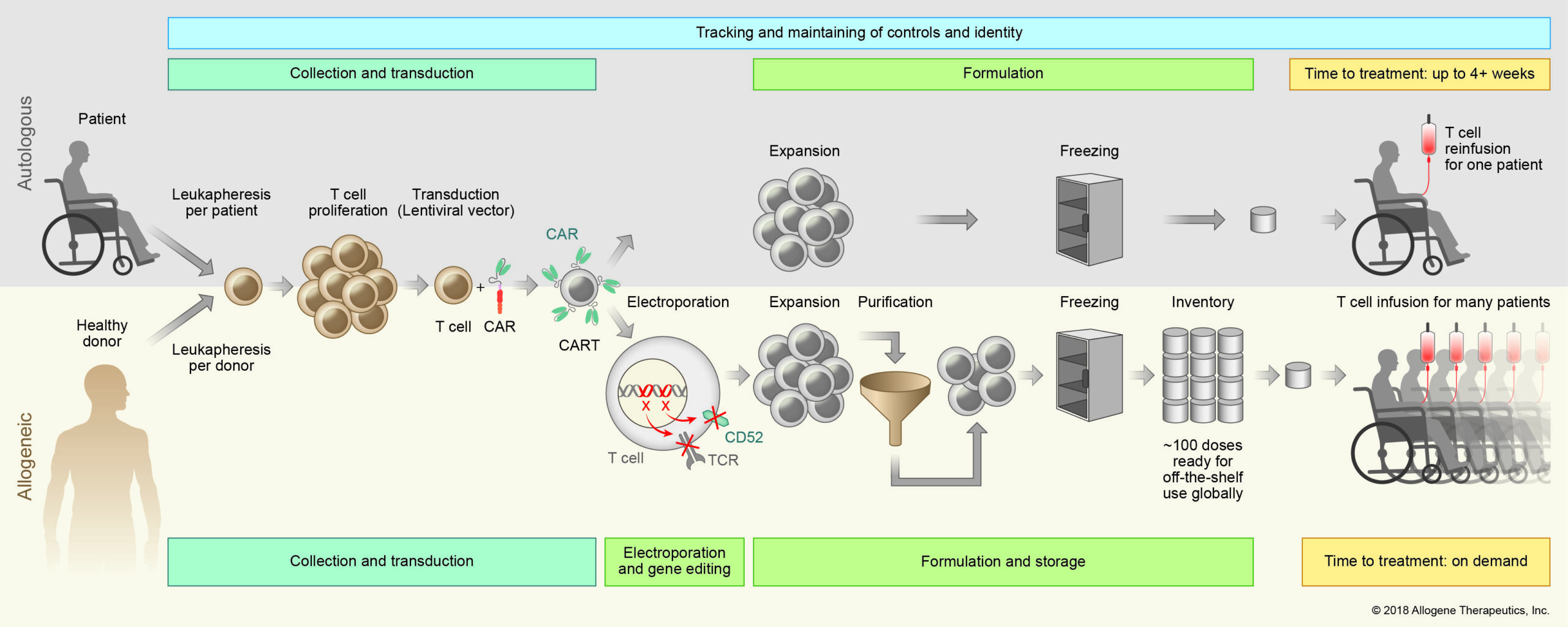
Our process for manufacturing our off-the-shelf AlloCAR T™ products first involves collecting white blood cells from healthy donors. The collected cells are then screened, tested, and shipped to a central processing facility, where the T cells are isolated and stored frozen, creating an inventory of healthy donor cells for manufacturing. This means that a larger portion of eligible patients, including those who are critically ill and have T cells that are difficult to harvest or expand, can potentially receive treatment, without having to undergo leukapheresis (a laboratory procedure in which a patient’s white blood cells are separated and the remaining blood cells and plasma are returned to the patient).
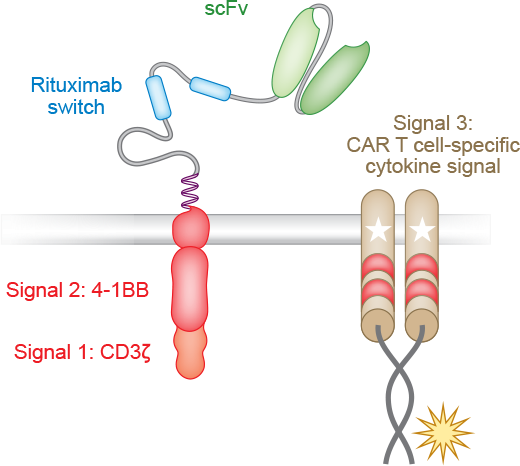
Next, the T cells are engineered to express CARs, which recognize certain cell surface proteins that are expressed in hematologic or solid tumors. ALLO-501A, our lead investigational product which utilizes our Alloy manufacturing process, targets CD19 for the treatment of relapsed or refractory (r/r) Large B Cell Lymphoma (LBCL). Our next two most advanced products are ALLO-715, which targets BCMA for the treatment of r/r Multiple Myeloma and ALLO-316 which targets CD70 and is currently being investigated for the treatment of clear cell renal cell carcinoma. These are just the first in a line of AlloCAR T™ products we plan to develop.
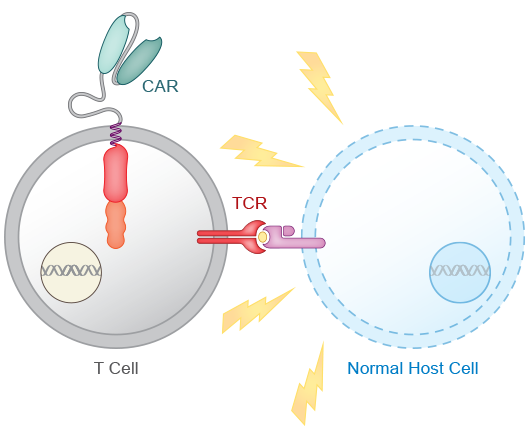
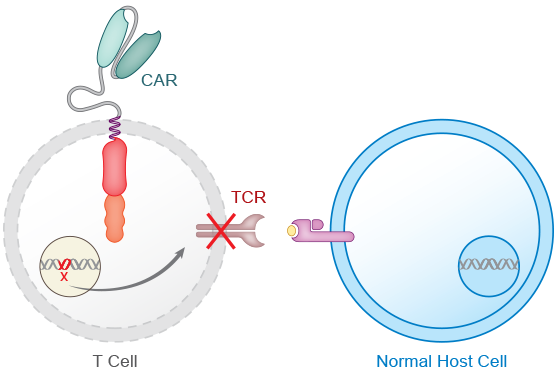
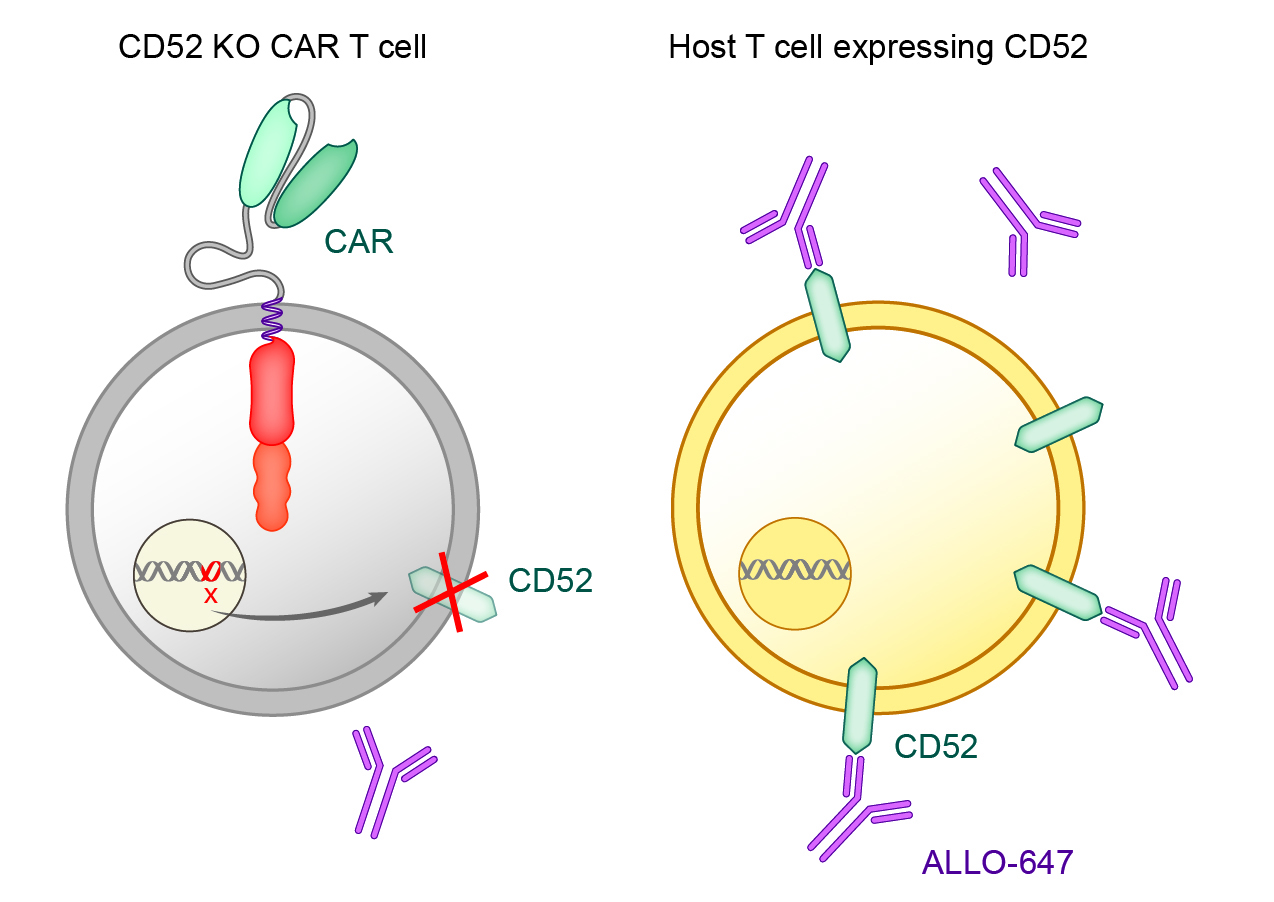
The next step in the process involves gene editing to reduce the risk of graft versus host disease (GvHD) and allogeneic rejection. A T cell receptor gene is knocked out to avoid GvHD. The CD52 gene is knocked out to render the CAR T product resistant to anti-CD52 antibody treatment. ALLO-647, our investigational anti-CD52 monoclonal antibody, can therefore be used to suppress the host immune system and potentially allow the AlloCAR T™ to stay engrafted to achieve full therapeutic impact.
The engineered T cells then undergo a purification step and are ultimately cryopreserved in vials for on demand delivery to patients.
ALLOGENE IS CREATING THE AlloCAR T™ PLATFORM FOR TOMORROW
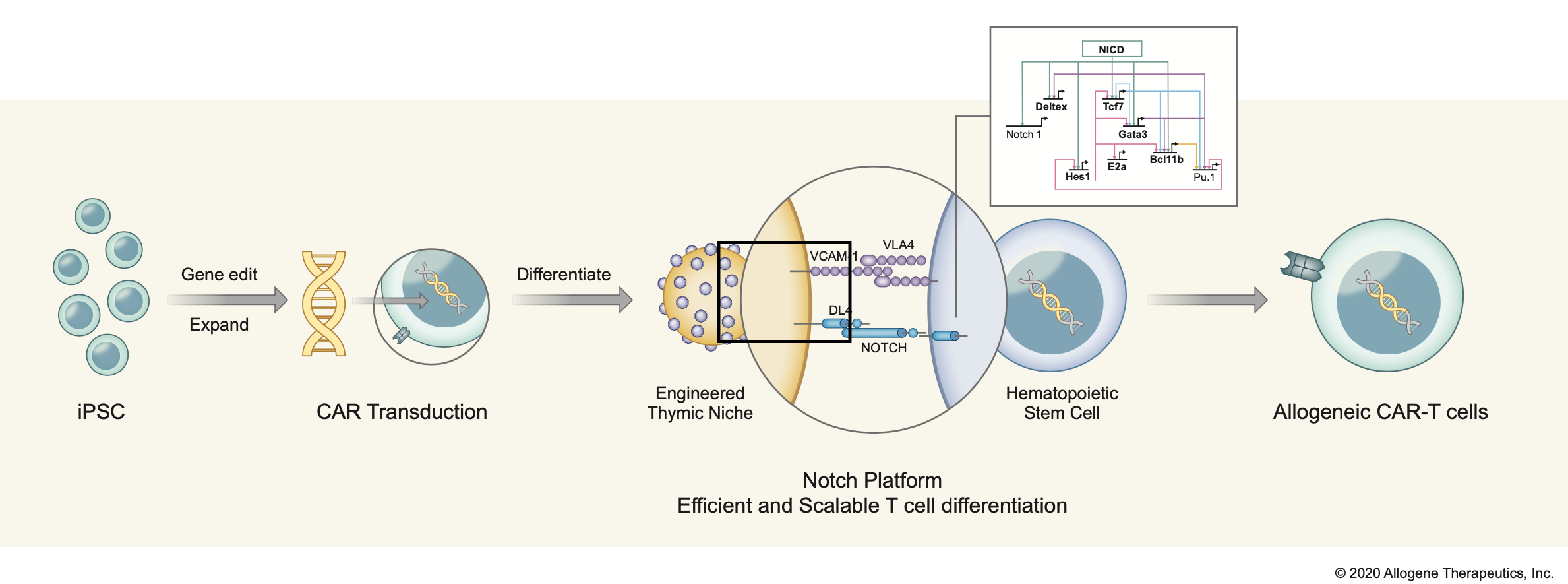
INDUCED PLURIPOTENT STEM CELLS (iPSCs)*
- Renewable starting-cell source
- Master cell bank of engineered
iPSCs - Proprietary T cell differentiation
technology
IMPROVING T CELL FITNESS
- TurboCARs™
- Manufacturing improvement
- Site-specific integration
PREVENTING GRAFT REJECTION
- Enhanced lymphodepletion
- Immune evasion
EXPANDING TARGET REPERTOIRE
- Target selection/validation
- CAR optimization
- Multitargeting CARs
*In collaboration with Notch Therapeutics.
STATE-OF-THE-ART, IN-HOUSE MANUFACTURING FOR ON-DEMAND AlloCAR T™ AVAILABILITY

Maintaining state-of-the-art, world-class manufacturing capabilities is at the core of our strategy to deliver readily available cell products faster, more reliably, and at greater scale. Our manufacturing facility, Cell Forge 1, located in Newark, California, in the eastern region of the San Francisco Bay Area, is designed for clinical and commercial manufacturing, analytical testing and distribution of cell products. This facility provides in-house quality assurance and quality control, per current good manufacturing practices (cGMPs), to ensure a reliable supply of AlloCARs™ for our clinical programs and commercial products, pending regulatory approval.

MODULAR, FLEXIBLE DESIGN
Our modular, 136,000 square-foot facility was designed for growth. It currently has three production suites, with the ability to accommodate five as we look ahead to bringing new therapies onto the market.

IN-HOUSE QUALITY CONTROL AND TESTING
We believe in the critical importance of understanding the quality of our investigational AlloCAR T™ products, which is why we operate the entire scope of product testing and maintain an open data strategy in-house.
COMMITMENT TO SUSTAINABILITY
Cell Forge 1 is a carbon-free facility that is fully powered by electricity, much of which will be delivered by the 2,400 solar panels on the rooftop.
CF1 received LEEDv4 Interior Design and Construction Gold certification.

COLLABORATIVE DESIGN
We included large windows throughout the facility to promote a culture of transparency and connection. Open workspaces, as well as the facility’s proximity to Allogene’s Headquarters, allow for regular cross-functional collaboration.