PIPELINE
OUR AlloCAR T™ PRODUCT PIPELINE TARGETS A VAST ARRAY OF TUMOR TYPES AND AUTOIMMUNE INDICATIONS
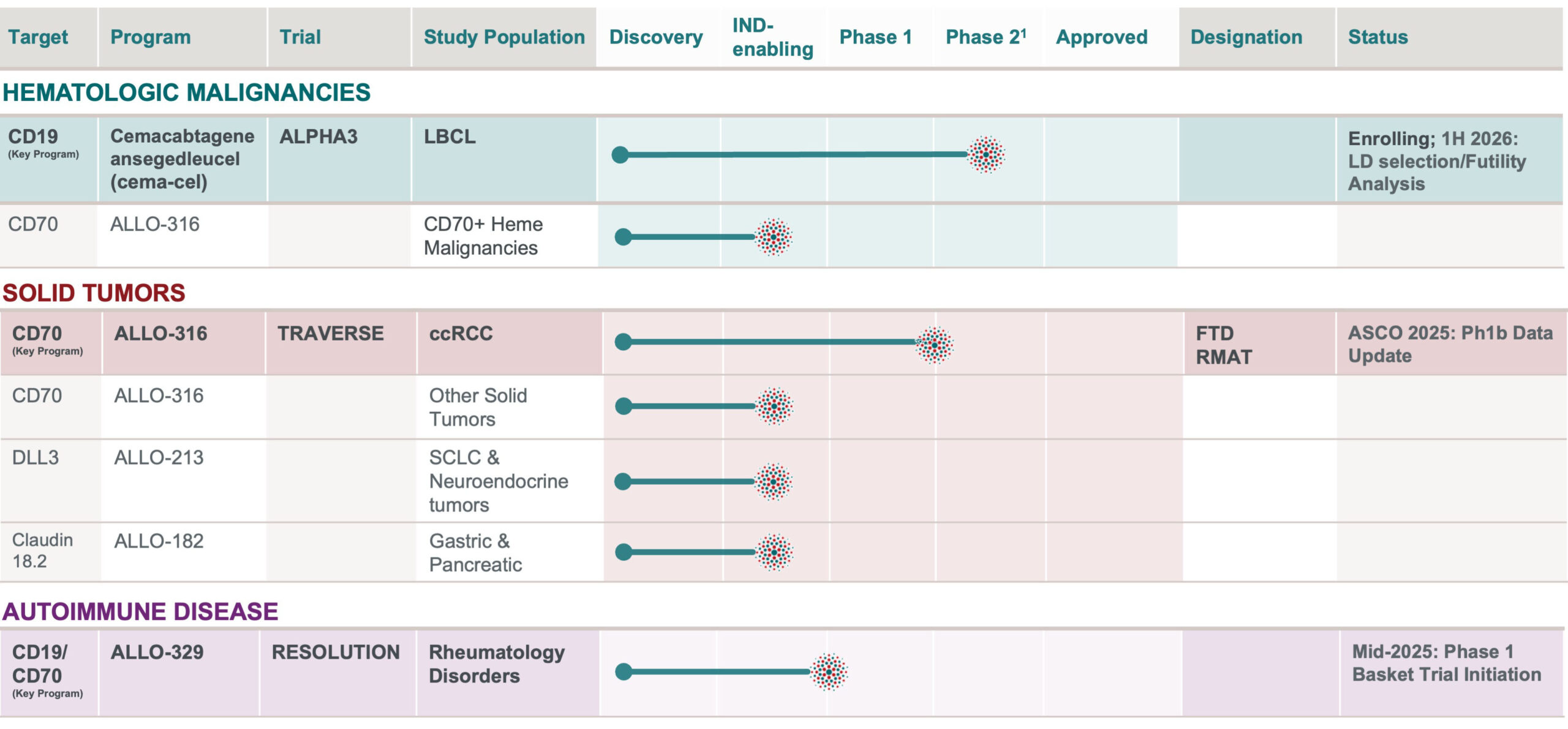
¹Phase 2 designed to be registrational
DATABRIEF
The DataBrief video series highlights the latest clinical findings from our investigational allogeneic CAR T cell (AlloCAR T™) products.
ALLOGENE’S EXPANDED ACCESS POLICY
“Expanded Access” refers to the use of an investigational product outside of a clinical trial for potential treatment of a serious or life-threatening condition. Allogene Therapeutics is developing allogeneic chimeric antigen receptor T cell (AlloCAR T™) products for patients with blood cancers, solid tumors and autoimmune disease. Consistent with our commitment to bring innovative, safe, and effective products to patients, we are focused on enrolling and conducting the clinical trials necessary to gain regulatory approvals to make our products broadly available as quickly and safely as possible. Participation in one of our clinical trials is the best and preferred route to access these investigational products. Allogene does not currently have any active Expanded Access protocols nor do we currently provide access to our investigational products on an Expanded Access basis. We encourage patients interested in our investigational products to learn more about our ongoing studies by visiting clinicaltrials.gov.
Treating physicians may request information about Allogene’s Expanded Access policy by contacting our clinical trials team:
By phone
415-604-5696
By email
clinicaltrials@allogene.com
Allogene will respond to inquiries within 5 business days from receipt.
In the event Allogene decides to consider making one or more of its investigational candidates available for patients who have a serious or life-threatening condition through an Expanded Access program, general criteria to be considered may include: availability of alternative products, the potential risks and benefits to the patient, adequate supply, and potential interference with Allogene’s ongoing clinical trials. All requests would be evaluated on a case-by-case basis in a fair and equitable manner.
This policy shall not serve as a guarantee of access to any specific investigational candidates by any individual patient. Allogene reviews its policies from time to time to ensure conformity with applicable laws and regulations. We reserve the right to revise this policy at any time.
