ALPHA3
NOW ENROLLING
The ALPHA3 trial aims to embed an investigational allogeneic CAR T product as part of the first‑line (1L) treatment regimen in LBCL
CEMACABTAGENE ANSEGEDLEUCEL (CEMA-CEL) IS AN ALLOGENEIC CAR T PRODUCT BEING STUDIED AS PART OF 1L TREATMENT FOR PATIENTS WITH LBCL/DLBCL WHO ARE LIKELY TO RELAPSE
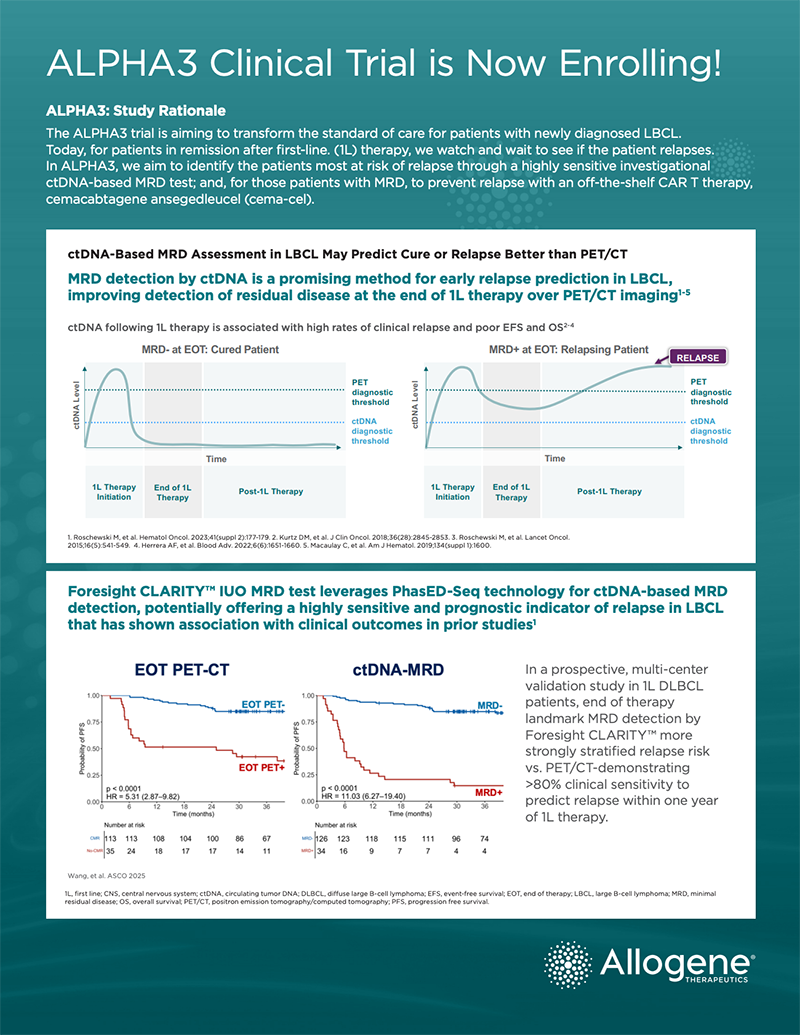
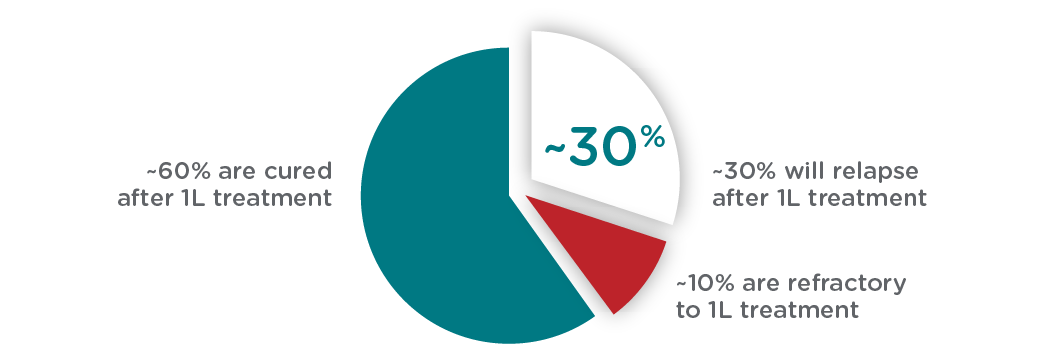
Over 60,000 patients are expected to be treated for LBCL, including DLBCL, annually in the US, the EU, and the UK. While first-line (1L) chemoimmunotherapy (R-CHOP) is effective in most patients, 1 in 3 will relapse and require subsequent treatment. Currently, no diagnostic test exists to identify those patients whose disease will relapse.
AMONG PATIENTS TREATED WITH R-CHOP
ALPHA3 STUDY DESIGN OFFERS UNIQUE FEATURES
- NO COMPETITION: Creates a unique treatment option to become the "7th cycle" in 1L treatment
- EXPLORING A NEW STANDARD-OF-CARE: Compares treatment with cema-cel, which in Phase 1 studies has demonstrated a 58% complete remission (CR) rate in relapsed/refractory LBCL, against observation (the current standard-of-care)
- SELECTED PATIENT POPULATION: Enrolls only patients at high-risk of relapse when their disease burden is low, aiming to maximize the efficacy of CAR T therapy and opportunity for outpatient treatment
- ACCESIBLE TO PATIENTS: Allows for CAR T in community-based cancer centers where ~80% of LBCL patients receive care, greatly expanding patient access
FIRST PROSPECTIVE TRIAL TO INCORPORATE INVESTIGATIONAL FORESIGHT CLARITYTM MRD TEST
The presence of minimally residual disease (MRD) remaining after therapy is predictive of relapse, yet currently there are no tests available that have the accuracy/performance to reliably detect residual disease at the end of 1L treatment in LBCL, including DLBCL. The ALPHA3 trial is the only study using a newly developed investigational test—Foresight CLARITY™ powered by PhasED-Seq™ (Foresight Diagnostics)—to screen patients who are likely to relapse after 1L treatment for enrollment in the trial. Leveraging CLARITY’s ultra-sensitive MRD technology, cema-cel will be administered as a one-time infusion immediately upon detection of MRD at the completion of six cycles of R-CHOP or other standard 1L chemoimmunotherapy regimen.
STUDY DETAILS
The pivotal Phase 2 ALPHA3 trial (N=240) will leverage a state-of-the-art, ctDNA-based investigational minimal residual disease (MRD) assay to identify patients whose disease is likely to relapse and randomize against the current standard-of-care (“watch-and-wait”) versus a single infusion of cema-cel.
Cema-cel in 1L consolidation LBCL can potentially change how patients with LBCL, including DLBCL and similar types of lymphoma are managed and provide revenue potential of ~$5B in the US, EU and UK.
DataBrief
Explore the ALPHA3 trial investigating cema-cel as part of 1L treatment for patients with LBCL/DLBCL who are likely to relapse.
CLINICAL TRIAL RESOURCES
These materials are designed for healthcare providers to support their discussions with patients about the ALPHA3 clinical trial.
Patient Video
Video: Learn About the ALPHA3 trial for LBCL/DLBCL.
R-CHOP, rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, prednisone.
This site is intended for people ages 18 and older.
AlloCAR T™ is a trademark of Allogene Therapeutics, Inc.
CLARITY™ and PhasED-Seq™ are trademarks of Foresight Diagnostics.
Foresight CLARITY™ Investigational Use Only (IUO) is limited by United States Law to investigational use.
Allogene’s investigational AlloCAR T™ oncology products utilize Cellectis technologies. The anti-CD19 products are developed based on an exclusive license granted by Cellectis to Servier. Servier, which has an exclusive license to the anti-CD19 AlloCAR T™ investigational products from Cellectis, has granted Allogene exclusive rights to these products in the U.S., all EU Member States and the United Kingdom. Foresight CLARITY™ IUO is for research use only. It is not intended for diagnostic procedures.