RESOLUTION
NOW ENROLLING
Exploring a next-generation allogeneic CAR T for the treatment of autoimmune diseases
NEXT GENERATION “OFF-THE-SHELF” DUAL‑TARGETED CD19/CD70 CAR T PRODUCT AIMS TO RESET THE IMMUNE SYSTEM AND INDUCE DURABLE DRUG-FREE REMISSIONS IN PATIENTS WITH LUPUS, MYOSITIS AND SCLERODERMA
Many patients with autoimmune diseases struggle with treatments that inadequately control disease or cause significant side effects. What is needed is a lasting therapy that safely resets the immune system without long-term immunosuppression.
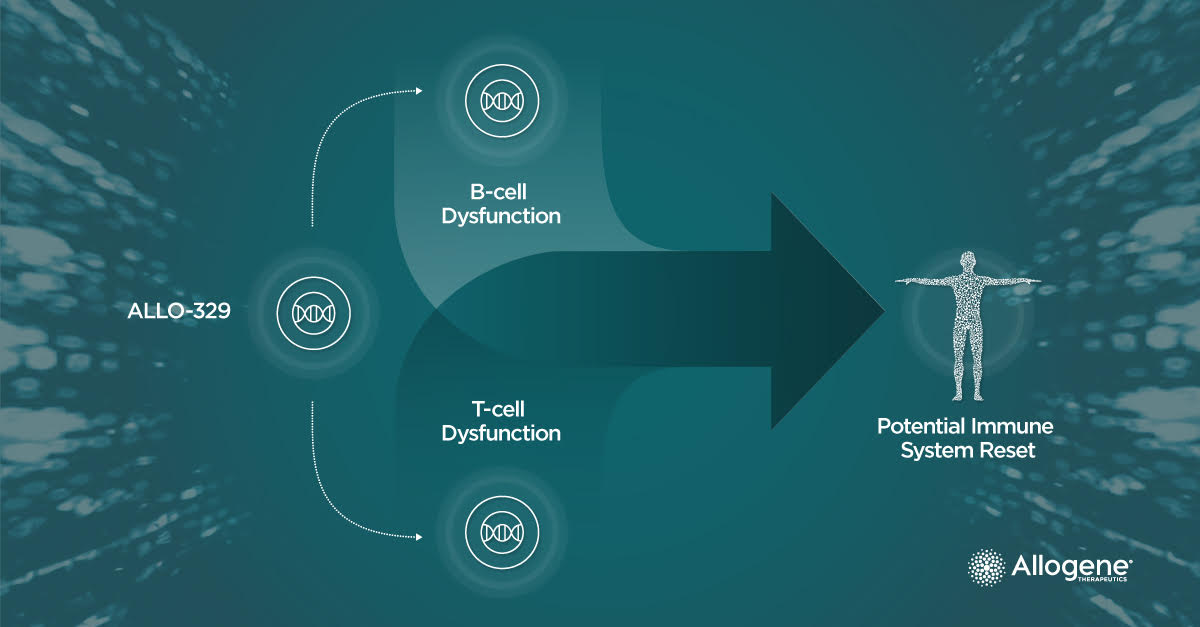
ALLO-329, a next-generation, "off-the-shelf" dual-targeted CD19/CD70 AlloCAR T™ product, incorporates Dagger®, Allogene’s proprietary technology designed to protect CAR T cells from host immune rejection. This innovation may eliminate the need for traditional lymphodepleting chemotherapy.
The Phase 1 RESOLUTION basket trial will evaluate the safety and preliminary efficacy of ALLO-329 in patients with lupus, myositis and scleroderma. Originally developed for cancer, CAR T therapy now holds promise as a one-time treatment capable of inducing durable, drug-free remissions in autoimmune disease.
RESOLUTION Basket Study Addressing Four Large Diseases
Estimated US Diagnosed Prevalence
Lupus (SLE)
330,000
Systemic Sclerosis
100,000
Lupus Nephritis
90,000
Myositis
70,000
By simultaneously targeting CD19+ B-cells and CD70+ activated T-cells, ALLO‑329 addresses multiple components of immune dysfunction that drive autoimmune disease. This approach aims to reset immune balance, achieve durable, drug-free remission and potentially broaden treatment options across a range of autoimmune conditions.
What Sets the RESOLUTION Trial Apart
What sets the Resolution trial apart is its bold goal: to reduce—or even eliminate—the need for lymphodepletion. Achieving this goal could mark a major leap forward in how CAR T therapy is delivered, streamlining the process and making it far more accessible to patients, especially women of childbearing age. This kind of advance, paired with the ability to deliver the product on demand, has the power to reach and benefit the more than half a million people living with autoimmune diseases in the U.S. alone.1
- A Dual-Targeted Approach: ALLO‑329 is an “off-the-shelf” CAR T therapy that uniquely targets both B cells (CD19) and T cells (CD70) to address key drivers of autoimmune disease.
- Reduce Toxicity from Lymphodepletion: Powered by Allogene’s proprietary Dagger® technology, ALLO‑329 is designed to resist rejection and may reduce or eliminate the need for traditional pre-treatment lymphodepletion. This innovation could simplify CAR T therapy and help expand its use in autoimmune diseases.
- Scalability Expands Patient Access: ALLO‑329 is an “off-the-shelf” CAR T therapy designed for immediate use as a one-time treatment for autoimmune diseases. Its ready availability enables faster patient access and aims to address the significant unmet need in autoimmune care.
1 Allogene Data on File
This site is intended for people ages 18 and older.
AlloCAR T™ is a trademark of Allogene Therapeutics, Inc.



